
Close


March 25, 2020
By Nicholas Del Re

Laboratory-grown diamonds created through chemical vapour deposition (CVD) and high pressure, high temperature (HPHT) processes are grown by a number of manufacturers who specialize in this field.
As these gemstones continue to find their place on the market, it is becoming increasingly important for retailers, designers, and other jewellery professionals to have reliable methods of detecting CVD diamonds from those that are natural—especially when the gem-cut end product of CVD crystals is visually the same as those of their natural counterparts. Disclosing the true origin of growth for diamonds, whether natural or created in a laboratory, is necessary to maintaining trust with customers and clients.
Fortunately, if one knows what to look for, subtle differences are indeed present between these two types of diamonds. Whether loose or in jewellery, CVD and HPHT laboratory-grown diamonds are detected and separated through rigorous process at Gemological Science International’s (GSI’s) laboratories; the use of carefully selected instruments by experienced staff members generate visual and spectral information that allows for this detection and separation.
When it comes to differentiating CVD and HPHT laboratory-grown diamonds from those that are natural, one of the most certain methods of separation is done through a combination of visual observations and photoluminescence.
Visual observations are achieved by using an imaging instrument, known as the DiamondView, which brings out structural growth characteristics and differences, along with variances in colouration.
This involves placing a diamond in an enclosed chamber while it is exposed to high-energy ultraviolet (UV) rays. Among the reactions seen are two kinds of luminescence called fluorescence and phosphorescence.
To give one an idea of the lower to higher energies used to excite the diamond by UV light, these are respectively: longwave (LW), shortwave (SW), and DiamondView (DV), with the wavelengths in nanometres (nm) as follows:
LW=365nm SW=254nm DV≈225nm
Fluorescence is the luminescence seen while being excited by UV light, while phosphorescence is luminescence seen occurring after the excitation of the UV light is cut off.
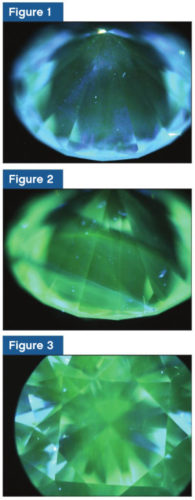
The telltale signs for differentiation of natural and laboratory-grown are from the colouration and appearance of structural features within the body of the diamond.
The other important test related to detecting and separating involves Raman instrumentation, which uses lasers that emit light at specific wavelengths. These are used to excite the internal atomic structure of the diamonds that, essentially, excite defects within the crystalline structure. This excitation results as photoluminescence; light emission from any form of matter after the absorption of energy, resulting in graphs that exhibit peaks which reflect the defects, and another peak that verifies it is a diamond. This, along aforementioned imaging, greatly helps one to differentiate natural diamonds from those that have been grown in a laboratory.
While performing these tests in GSI’s New York laboratory, researchers observed an unusual feature exhibited by a CVD laboratory-grown diamond.
The stone in question weighed more than two carats and was near colourless in full-spectrum daylight lighting. When placed in the DiamondView, the initial results were as expected for a laboratory-grown CVD diamond.
The resulting fluorescence exhibited a green to blue colouration (Figures 1 to 3). The different degree of colouration observed was the result of variable exposure to the UV it was subjected to. Also, the characteristic placement of colour in the structure seen was due to the growth process involved for CVD diamonds.
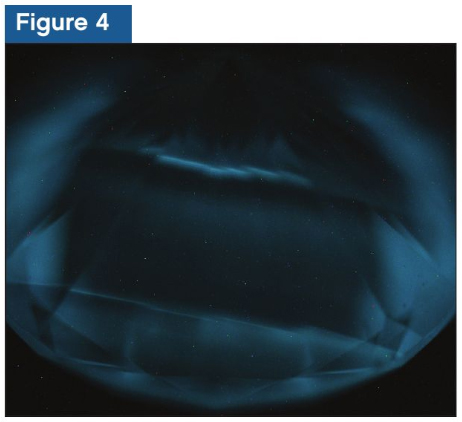
In addition to fluorescence, blue phosphorescence (Figure 4) was also observed.
These observations are fairly standard when viewing these diamonds under this condition; however, CVD diamonds can exhibit other phenomena under this or similar circumstances.
This brings us to a most interesting aspect of this particular CVD stone: it exhibited an unusual and dramatic change in colour after being taken out of the DiamondView instrument, going from near colourless to blue (Figure 5).
While the body colour of CVD diamonds is stable when viewed in daylight under normal conditions, it is known the colour may change if the conditions are altered (i.e. if the stone is exposed to UV light or heat). In these cases, however, the colour typically returns to its actual colour within 30 minutes to an hour of being in normal conditions and after direct exposure to full spectrum daylight lighting.
In regards to longwave and shortwave UV exposure, colour change is comparatively subtle, but becomes more dramatic as exposure and energy increase.
Although the colour of the CVD diamond reverts back to a normal stable colour, there are some caveats to be aware of. First off, while direct full spectrum lighting (e.g. a light box, preferably lacking or having minor UV radiation) would bring back the CVD diamond’s colour after about one hour, it would take two-and-a-half hours before this stone would start to return to its stable colour in an environment with standard ambient light conditions. (As a note: one should be mindful of the lighting environments that may vary in standard room-lit environments where gemstones are handled and viewed.)
The experiment continued once the colour changed to blue. The CVD stone was stored in parcel paper (read: a dark environment) for one week. When removed from the paper, it was discovered the stone was the same blue colour as it had been when placed in the paper. The research team was able to get the CVD diamond back to its original near colourlessness by exposing it to full spectrum daylight light box for about one hour.
Generally, diamonds created through CVD processes do not change to this extreme; still, most can exhibit subtler changes to varying degree. With this in mind, it is strongly recommended all laboratory-grown CVD diamonds be placed in a full spectrum light box for at least 30 minutes before attempting colour grading to ensure consistency with the colour grades given afterwards.
Although not the same, the occurrence is akin to one which happens in natural diamonds, classified as Chameleon. These Chameleon diamonds have unique colour-changing properties that make them desirable in the marketplace.
From a scientific (albeit simplified) viewpoint, the colour change observed is known as photochroism. This is a reversible transformation of a material’s colour due to exposure to electromagnetic rays, such as UV. (This is when the colour centres change its charge-state under optical illumination, such as UV.)
During this photochromic effect, basically, we have the electrons in defects within a CVD diamond being exposed to additional energy from a source as the UV. This causes the energy or charge state to change, which, in turn, affects the way colour is absorbed from the visible spectrum.
Returning to the noted change in this particular CVD diamond, we have defects from the silicon present in the growth process. This presence causes a complex of absorption peaks in the visible-to-near infrared region in the electromagnetic spectrum. The exposure to the energy of the UV causes a charge transfer in the silica vacancy centers that are present (one main vacancy centre is noted by the 737 nm absorption peak). While this is happening, it increases the absorption of the red in this region, causing the blue colouration to be seen. In addition to this being an occasional novelty for CVD diamonds, it may well find possible applications in quantum information processing.

Nicholas Del Re is chief information officer (CIO) of Gemological Science International (GSI). He has nearly four decades’ experience, working in research in research and gem identification departments of gemmological organizations, as well as serving in an R&D capacity with Industrial Diamonds. Del Re has presented at numerous functions within the jewellery industry, including the NY Mineral Club, where he has held office as secretary for many years. He can be reached via email at nickd@gemscience.net.